Advance Healthcare with
Top One Research Group

At Top One Research Group, participating in clinical studies means more than access to expert care, it’s a chance to create meaningful change in your community and beyond.
Whether you're exploring new treatment options or helping move science forward, your involvement makes a real impact. We’re committed to improving lives through inclusive, community-driven research across the United States.
Join us today, and be part of something bigger.

Medical Trials That Improve
THE MEDICAL INDUSTRY
Explore medical trials shaping a healthier future. Join us for an immersive experience supporting groundbreaking healthcare innovations.
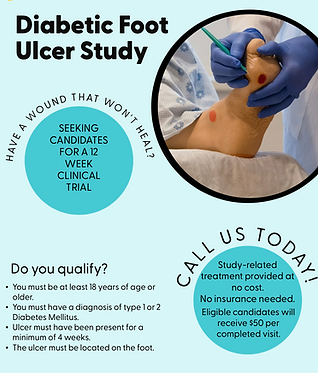
Discover If You Qualify
FOR A CLINICAL TRIAL
Find out if we have a clinical trial for you. Give us a call today to speak to a research staff member and find out which program you would qualify for.
High-Quality
PHYSICIANS AND STAFF
We have board-certified physicians that will provide you exceptional care with an overwhelmingly strong desire to help.

Enrolling Studies